Once a chemist has seen and understands potential for the application of metal scavenging and reduction of classical iterative metal migration steps, the next question I usually receive, relates to how best to apply them to a reaction.
Since metal scavenging is all about saving time, being more efficient, and hitting hard to reach purity goals, we want to deploy it most effectively for the polishing or clean up purification steps that we are aiming for. There are currently schools of thought that support the notion of purging impurities naturally in telescoped processes or removing them at the first opportunity in case of downstream side reactions, especially if they are based on metal catalysts, so we wanted to look into that more. We focused out study on the powerful metal scavenger ISOLUTE® Si-TMT, (Figure 1).
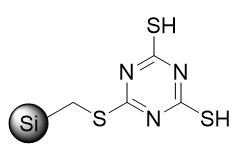
Figure 1. ISOLUTE® Si-TMT structure
So, for this experiment we went back to basics and used a very familiar and predictable Suzuki reaction, Figure 2.
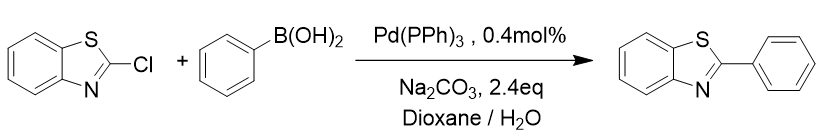
Figure 2. Model Suzuki reaction of popular benzothiazole starting material and boronic acid. I’ve color coded the various ‘modules’ of the workflow that we undertook.
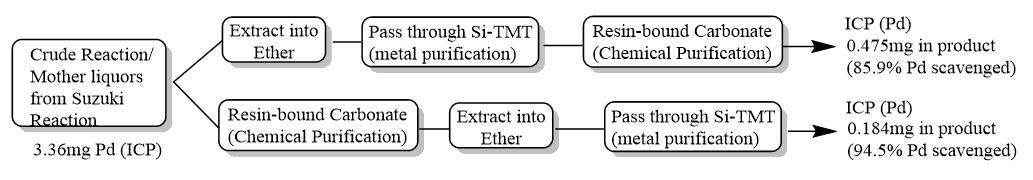
Loosely speaking, the reaction is typically followed by a workup, extraction, and purification of both chemical mix and metal. For simplicity, we used a resin-bound carbonate strategy for the chemical purification of excess boronic acid so we had the opportunity to vary where we employed the metal mitigation stage. (In this way, we have a certain orthogonality, and the steps essentially stand alone in terms of their function and activity).
Just after the reaction we recorded 3.36 mg of palladium in our sample. In the first tranche of experiments, we extracted the organic component into ether and passed that through a small plug of Si-TMT metal scavenger. Then we removed excess boronic acid using Biotage® MP-Carbonate and sent the results off for elemental analysis, finding 0.475 mg palladium.
Similarly, in tranche two, we applied the MP-Carbonate first to remove the boronic acid, extracted the product into ether, and then applied that to a plug of the Si-TMT metal scavenger. Analysis of that sample showed a palladium content of 0.184 mg.
Both reactions were identical in terms of the work up steps applied, however, the scavenging results were very different. We saw a significant enhancement of metal scavenging efficiency when the scavenging step was applied to the later stages of the reaction. Metal scavengers are designed to be added, stirred, and removed, or act as a fixed bed for fast throughput polishing steps. Also, it was reassuring to see that when they are applied later in a reaction, we saw ~10% increases in efficiency in metal scavenging from 85% to over 95%. This success was using the same system, scavenger amount, ligand, and target metal for removal and processing steps. Certainly not something to balk about or ignore, as that’s the difference between pass and fail!
In our previous blog post our in-house expert discusses how metal scavengers can be an alternative to recrystallization for removing metals. Follow the link to read it now: Learn More

 Organic Workflow
Organic Workflow Peptide Workflow
Peptide Workflow Scale-Up Flash Purification
Scale-Up Flash Purification  Sample Preparation
Sample Preparation Biomolecule Purification
Biomolecule Purification Oligo synthesis
Oligo synthesis Scavengers and Reagents
Scavengers and Reagents Service & Support
Service & Support Accessories & Spare parts
Accessories & Spare parts Investors
Investors Reports & News
Reports & News The Share
The Share Corporate Governance
Corporate Governance Calendar
Calendar Sustainability
Sustainability Our Offering
Our Offering Our History
Our History Our Locations
Our Locations Leadership
Leadership