Now we have a screening candidate, we just need to scale up our work, it is always advisable to initially to scale in factors of ~10 in our experience, although for well-used processes and repetitive batch processing, it is possible to screen grams and go straight to Kgs.
Recall, metal scavenging is not something we want to need to spend too much time on, the real focus of our work is to get product free of metals as quickly and as effectively as possible.
Luckily this type of scale up is robust, so we’ll shoot from the hip and choose a 50x scale up factor in our experiment. The screening cartridge in the previous experiment contained 1g of scavenger, so we chose a 50g cartridge (one of our SNAP purification cartridges that we filled with metal scavenger).
Since the screening experiment was a one-pass process, we wanted to retain that level of efficiency, so factors such as residence time were likely to be important to fix-on. Measuring the time it took for the Pd solution to pass through, we calculated the linear flow rate of the system was 0.8cm/min. That’s pretty slow, but the screening was processes under gravity, we were not pumping this through, so we made a mental note that this factor could potentially be improved in further optimizations. But our goal wasn’t to optimize just yet, first step is to demonstrate a scalable robust process, mimicking an efficiency of metal scavenging that was seen in our earlier screening experiments.
Using the following formula we can map a flow rate used for a screening experiment to a corresponding flow rate in virtually any proposed scaled up container – useful if we don’t yet know what scale we will eventually be working at. These calculations are based on the same rock-steady science used in flash purification scale up, we just add some common sense to the principles when it comes to the kinetics of binding.
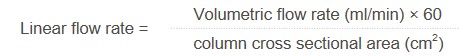
Resolving our 1g screening plug to a linear flow rate, and then transferring that linear flow rate to a larger vessel, we can work out the new required volumetric flow rate for the scaled-up example. This is particularly helpful when we consider that we can just dial that required flow rate into the pump controller on scale up, and the kinetics and efficiency will transfer accordingly. Additionally, if you have an automated flash system, you could potentially just program a method, and get the system to plot a changing UV absorbance at the same time.

So, our calculated flow rate is 15mL per minute (I’ll talk more about that later in the optimization section), we’ve chosen Si-TMT, and want to do a one-pass cartridge style clean-up process. (This is not the only type of scavenging possible, we could easily have added into a round bottom flask and stirred until aliquots showed the palladium had been removed). But here, we’ll equilibrate a 50g fixed bed similarly using 3CV of ethyl acetate (as long as the bed is wet, it doesn’t really matter how many CV are used).
When we replay those conditions using the same concentration (but of course larger volume and amount of Pd) of stock solution, here’s what we saw:
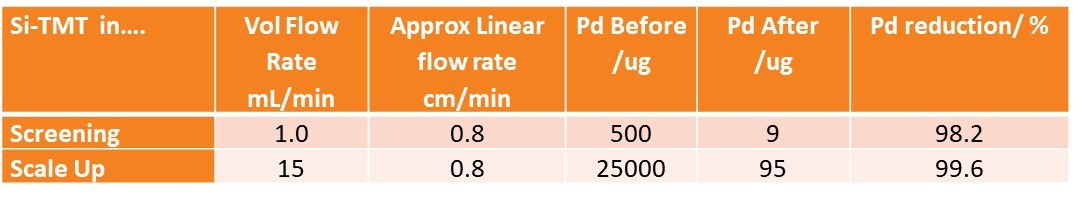
Remember that the scale up parameters encoded in this scale up experiment do not give an ABSOLUTE metric for the removal of palladium, this is where a lot of people go wrong. In doing it this way, we’ve created an experiment which recreate and enjoy the RELATIVE efficiency, meaning if the screening experiment removed 98% of the soluble palladium, then the scale-up experiment would be expected to remove 98% of the soluble palladium. [Actually, for reasons of better packing and more accurate measurement of contact time on scale up, we found our efficiency increased slightly, to 99.6% Pd removal].
Fast forward to what these numbers may mean in practice. 25000 micro grams of Pd, represents 0.235mmol Pd, which would be enough to deal with a 47mmol reaction, run at the typical 0.5mol % level - typical for a catalyst. Assume a small module or API with a molecular mass of ~350, then we’re already talking about mitigating Pd from a 16g scale chemical synthesis. Not bad considering this was one pass and implemented in a matter of minutes and that fixed bed had more capacity in it, so could have been pushed much harder.
Conclusion
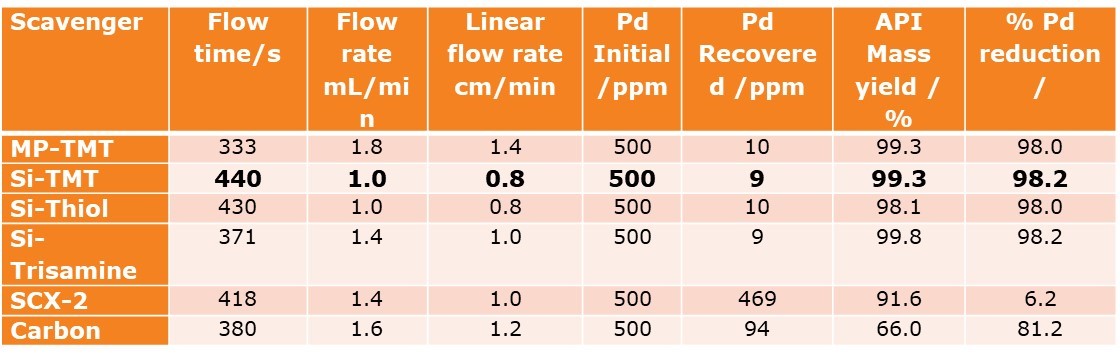
Si-TMT is one of the most powerful scavengers we know, by virtue of the trimercaptotriazine ligand, and is often applied as the nuclear approach when the job of Palladium removal is difficult, or when there may be inherent variability in an input process stream. Those other candidates in the screening may have been just as viable, and would have worked also, but if we look at the efficiency of scavenging, compared with the % of product that may be lost (which we obviously want to minimize), our selection quickly slims down to 3 neutral metal scavengers based on some tried and tested ligand – palladium interactions.

 Organic Workflow
Organic Workflow Peptide Workflow
Peptide Workflow Scale-Up Flash Purification
Scale-Up Flash Purification  Sample Preparation
Sample Preparation Biomolecule Purification
Biomolecule Purification Oligo synthesis
Oligo synthesis Scavengers and Reagents
Scavengers and Reagents Service & Support
Service & Support Accessories & Spare parts
Accessories & Spare parts Investors
Investors Reports & News
Reports & News The Share
The Share Corporate Governance
Corporate Governance Calendar
Calendar Sustainability
Sustainability Our Offering
Our Offering Our History
Our History Our Locations
Our Locations Leadership
Leadership